Virus Testing
While human and animal cells are not per se harmful during routine handling, cell cultures may be contaminated with other organisms from elevated risk groups, notably bacteria or viruses. All cell lines in the open collection are tested for a panel of human pathogenic viruses by polymerase chain reaction (PCR).
Download the submission form.
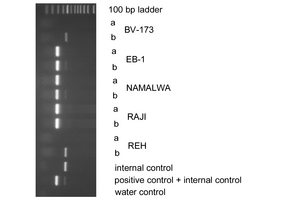
These investigations are important for the risk assessment regarding the handling of cell cultures, because several countries legally require tests for specific human pathogenic viruses to determine precise safety levels. The following PCR assays are performed at the DSMZ and can be requested as service:
- Hepatitis B (HBV): In case of an infection, the 3.2 kbp HBV genomes either occurs as extrachromosomal episomes in the nuclei or integrated into the chromosomes of the host cells. Genomic DNA is isolated and a PCR specific for the various HBV subtypes is performed (including an internal control DNA). If an infection is detected, conditioned cell culture supernatant is harvested, cells and cellular debris is removed by centrifugation and the supernatant is subsequently ultra-centrifuged to obtain potential active viruses. DNA is extracted from the pellet and analyzed for HBV sequences by PCR.
- Hepatitis C (HCV): HCV is an RNA virus detectible by RT-PCR; total RNA is isolated from the cells, reverse transcribed to cDNA with random hexamers and PCR performed with HCV specific oligonucleotides (including an internal control DNA).
- Human herpesvirus type 4 (HHV-4, also called Epstein-Barr virus [EBV]): virus episomal DNA is isolated together with cell line genomic DNA and primers applied to detect HHV-4 specific sequences; if testing positive, further investigations might be necessary to determine the production of active viruses, the potential of the cells to induce the replication of the virus, or whether the infection is latent; PCR is performed including an internal control DNA (figure above); in addition to safety assessment, the detection of HHV-4 is important to distinguish tumor cell lines from virally immortalized (normal) B-lymphoblastoid cell lines. The lytic replication cycle associated with the production of active viruses can be determined by detecting the immediate-early gene product ZEBRA by Western blotting.
- Human herpesvirus type 8 (HHV-8): this virus occurs predominantly in primary effusion lymphoma (PEL) cell lines and is detected like with HHV-4 applying specific primers for HHV-8 sequences.
- Human immunodeficiency virus type 1 (HIV-1): proviral DNA sequences of this retrovirus are detected in the genomic DNA of the cell cultures including an internal control plasmid; the detection of the proviruses is particularly important for cell lines intended for use in genetic modification experiments using retroviral vectors.
- Human immunodeficiency virus type 2 (HIV-2): proviral DNA sequences of this retrovirus are detected in the genomic DNA of the cell cultures; due to sequence variations this virus cannot be detected simultaneously with HIV-1.
- Human T-lymphotropic virus type I and II (HTLV-1/-2): these retroviruses are similarly detected as with HIV-1 applying mixtures of oligonucleotides to detect proviral sequences in the cell line genomes; an internal control DNA has been developed to monitor sensitivity.
- Human papilloma viruses (HPV): the subtypes of this integrating virus species are detected in cell line genomic DNA using different primer mixtures.
- Squirrel monkey retrovirus (SMRV): this retrovirus has been described to infect diverse cell lines of different species, including human, and may be transferable between cell cultures; two different primer pairs are applied to detect proviruses in the genomic DNA of the cell lines.
- Xenotropic murine leukemia virus (XMLV): this rodent-associated retrovirus was found to contaminate human and other in vitro cell lines; the provirus is detected like with the other retroviruses in the genomic DNA of the cell lines.
Growing cell cultures from which we can harvest the conditioned medium and cells are best suited for virus detection. Adherent cell lines should be semi confluent and suspension cultures should have reached half their maximal cell density. Prior to shipment, culture vessels should be completely filled with medium and tightly closed (seal with Parafilm to obviate leakage). Shipments should be consigned early in the week. Please notify us beforehand. IMPORTANT: The DSMZ is forbidden to handle cell cultures infected with organisms higher than risk group two (RG2).
Detailed information on the methods can be found in the following publications:
- Uphoff CC, Denkmann SA, Steube KG, Drexler HG: Detection of EBV, HBV, HCV, HIV-1, HTLV-I and -II and SMRV in human and other primate cell lines. J Biomed Biotechnol, Vol. 2010: Article ID 904767, 23 pages (2010). PubMedID 20454443
- Uphoff CC, Lange S, Denkmann SA, Garritsen HS, Drexler HG: Prevalence and characterization of murine leukemia virus contamination in human cell lines. PLoS One 10: e0125622 (2015). PubMedID 25927683
For pricing, please refer to our list.
For technical questions contact
Team Virus Testing |
Questions related to ordering
Team Sales |

